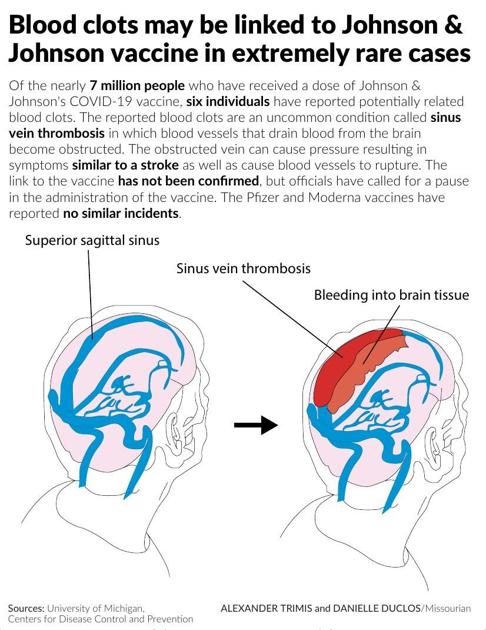
After the FDA and CDC released a joint statement regarding the Johnson & Johnson (Janssen) COVID-19 vaccine Tuesday, vaccinations were quickly suspended.
The organizations had begun to review data involving six cases of a “rare and severe” type of blood clot that had appeared in people after they’d received the single-shot Johnson & Johnson vaccination. The condition, called cerebral venous sinus thrombosis, or CVST, was coupled with low levels of blood platelets.
No confirmed link to the vaccine has been found, but the CDC and FDA, as well as Missouri’s DHSS, were advising to pause out of an “abundance of caution.”
The people who were affected by the condition were all female and between the ages of 18 and 48. The symptoms of CVST occurred six to 13 days after vaccination. One woman died from the condition and another was hospitalized.
Roughly 6.8 million Americans have received the J&J vaccine. That means the risk of the clotting complication is less than 0.00001%.
That’s comparable to the rates observed with the U.K. and E.U.’s AstraZeneca vaccine. Of 34 million doses administered, there were 169 reports of CVST, according to Reuters. This makes for about a 0.0005% rate, or 0.5 per 100,000.
Both AstraZeneca and Johnson & Johnson are adenovirus-based vaccines, which function more traditionally than mRNA vaccines. Both require only a single dose.
Missouri was among the states that paused vaccinations Tuesday, and a new standing order for the J&J vaccine was issued by the state’s Department of Health and Senior Services.
The federal government also halted administration of the vaccine by the U.S. military and federally run sites, as well as CVS and Walgreens pharmacies.
At a news conference Tuesday, Janet Woodcock, the acting commissioner of the FDA, said the pause was only expected to last “a matter of days,” although it depended on “what we learn in the next few days.”
CVST, according to Johns Hopkins, is a blood clot that forms in the brain’s venous sinuses. This prevents blood from draining out of the brain and may break and leak blood into brain tissues, causing a hemorrhage.
Normally, a blood clot would be treated with an anti-coagulant medication such as heparin. However, CVST requires much different treatment.
“Part of the reason for pausing today is because they want to inform health care providers,” said Margaret Day, co-chair of MU Health Care’s vaccine committee. “It’s really important to not treat that with the usual blood thinners. It’s accompanied by low platelets, which can cause people to have bleeding complications.”
The CDC and FDA announced in their statement that they planned to meet to look more closely at the patients’ cases and assess their possible significance. The organizations also wanted to ensure health providers were aware of the possibility of CVST and could successfully treat it.
Little is known about whether or when the vaccine will be reintroduced, but providers have been instructed to hold on to their doses for the time being.
The decision to pause J&J vaccinations prompted backlash, some of which was due to the fact that J&J doses are easier to ship, require just one shot and do not require super-cold storage like the mRNA vaccines. These factors have made the J&J vaccine a better fit for rural communities, the poor and people without shelter.
According to a study conducted by the Boston Collaborative Drug Surveillance Program, CVST occurs in 2.7-40 people per 100,000 who use oral contraceptives. That’s as much as 132 times the observed results of Johnson & Johnson’s COVID-19 vaccine.
Other conditions, such as pregnancy or Crohn’s disease, increase the risk of developing CVST. The most notable one, however, is COVID-19. COVID-19 patients have roughly a 30% risk of developing a blood clot and a 0.0045-0.02% chance of developing CVST specifically. That’s 66 times the risk for J&J vaccine recipients.
Contracting COVID-19 carries a greater risk of CVST than being vaccinated for it. “We do hope that the message is really clear that vaccines are far and away safer than getting the COVID infection,” Margaret Day said.
“I see this as a process that is working the way that it should,” Day said. “Certainly we don’t want any adverse effects for anyone. But we are finding out about these safety signals, and our regulatory agencies are investigating them thoroughly. It took 7 million vaccinations with Johnson & Johnson for us to find this incredibly rare, adverse event.”
Day, as well as the DHSS and CDC/FDA statements, urged those who have received the J&J vaccine within the past three weeks to be alert for the symptoms but not to become overly stressed or anxious. A severe headache, abdominal or leg pain and shortness of breath warrant contact with a health care provider.
"low" - Google News
April 14, 2021 at 09:00AM
https://ift.tt/3a9zgYZ
Clot risk very low from Johnson & Johnson vaccine - Columbia Missourian
"low" - Google News
https://ift.tt/2z1WHDx
Bagikan Berita Ini














0 Response to "Clot risk very low from Johnson & Johnson vaccine - Columbia Missourian"
Post a Comment